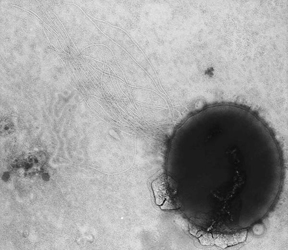
Methanococcus maripaludis strain C5, strain C6 and strain C7 William B. Whitman
Summary Methanococcus maripaludis is a rapid growing hydrogenotrophic methanoarchaeon common in salt marshes, marine and estuarine environments in the Southeastern U.S.A. and probably elsewhere. It is obligately methanogenic; H 2 and formate serve as electron donors. Acetate, methanol and methylamines are not substrates for methanogenesis. Alcohols including isopropanol are not substrates for methanogenesis. All strains grow autotrophically in mineral medium. Amino acids and acetate are frequently stimulatory for growth. Nitrogen sources include ammonium, N 2 gas and alanine. The ability to use alanine is distinctive for members of this species. Sulfur sources include sulfide and elemental sulfur. Storage materials include glycogen. It is an irregular coccus, ~1.0 µm in diameter during balanced growth (see Figure 1). Cells from older cultures or colonies are extremely irregular. Cells lose integrity during Gram staining and lyse completely within 10 s when suspended in either distilled water or 0.01% sodium dodecylsulfate. It is motile, by means of polar tufts of flagella and obligately anaerobic. It is a mesophile, with a temperature optima of about 35-40 o C. The pH optimum for growth is between 6 and 8. NaCl is required for growth, with optimal concentrations of 0.5-4% (w/v). The mol% G+C of the DNA is 33.
Further Descriptive Information Cell morphology . In growing cultures, cells are slightly irregular and uniform in size, between 0.9 and 1.3 µm in diameter. Pairs of cells are common. In stationary cultures, colonies or enrichment cultures, cell shape is very irregular, and large cells up to 10 µm in diameter are observed (Jones et al., 1977). In wet mounts, a few cells in a preparation may slowly swell and burst. Cells on the edge of a slide where drying may occur are much larger, less irregular, and more transparent than cells from the center of the slide. Cells from older cultures are mechanically fragile and rupture during vigorous stirring or upon harvesting by some continuous centrifugation devices. Cells are also osmotically fragile, and they lyse rapidly in distilled water. Cell integrity is maintained in 2% NaCl (w/v). Cells lyse rapidly in 0.01% sodium dodecylsulfate and contain a protein cell wall or S-layer. Growth conditions . All strains grow rapidly on H 2 + CO 2 or formate in pressurized culture tubes (Balch and Wolfe, 1976). Under optimal conditions, generation times are <3h even in completely mineral medium. Acetate, methanol and methylamines are not substrates for methanogenesis. All strains that have been tested are unable to utilize alcohols such as ethanol, isopropanol, isobutanol, and cyclohexanol as electron donors for CO 2 reduction (Zellner and Winter, 1987). M.maripaludis will grow in mineral medium with sulfide as the sole reducing agent and carbon dioxide as the sole carbon source (Whitman et al., 1986). Under these conditions, acetate and Casamino acids are frequently stimulatory, depending upon the strain. In addition to NaCl, high concentrations of magnesium salts are stimulatory or required for growth (Whitman et al., 1982, 1986; Corder et al., 1983; Jones et al., 1983a). Selenium is stimulatory to growth, and media contain high levels of iron, nickel, cobalt, and tungstate because they required or stimulatory for closely related organisms (Jones and Stadtman, 1977; Whitman et al., 1982). Sulfide or elemental sulfur are sufficient as sulfur sources. Cysteine, dithiothreitol and sulfate do not substitute for sulfide (Whitman et al., 1982, 1987). Some strains also utilize thiosulfate as a sulfur source (Rajagopal and Daniels, 1986). Ammonium, N 2 gas and alanine are nitrogen sources. (Whitman, 1989). Chemotaxonomic markers. A number of compounds are useful chemotaxonomic markers. The core lipid is composed of archaeol and hydroxyarchaeol (Koga et al. 1998). The polar head groups are composed of glucose, N-acetylglucosamine, serine and traces of ethanolamine. The most abundant polyamine is spermidine (Kneifel et al., 1986). The compatible solute common in the methanothermococci, ?$-glutamate, has not been detected (Robertson et al., 1990). Antibiotics and other inhibitors . Like other methanogens, methanococci are generally resistant to low concentrations of many common antibiotics (Jones et al., 1977). Some antibiotics which are inhibitory at low concentrations are: adriamycin, chloramphenicol, efrapeptin, leucinostatin, metonidazole, monensin, pleuromutilin, pyrollnitrin, and virginiamycin (Elhardt and Böck, 1982; Böck and Kandler, 1985). Methanococci are also sensitive to low concentrations of organic tin-containing compounds such as: phenyltin, tripropyltin, and triethyltin (Boopathy and Daniels, 1991). The methanococci have not been reported to be associated with disease . Habitats . The type strain of M. maripaludis was isolated from salt-marsh sediments near Pawley's Island, South Carolina (Jones et al., 1983a). Additional strains have been isolated from salt-marsh sediments in Georgia and Florida (Figure 2; Whitman et al., 1986; Keswani et al., 1996). Although they are not the most abundant H 2 -utilizing methanogens in these sediments, their rapid growth allows them out grow the more abundant species during enrichments with H 2 +CO 2 . Genetic systems. The mesophilic methanococci are one of the few groups of methanogens and archaea with a robust genetic system. Historically, the study of both methanogens and archaea has been greatly limited by the absence of genetics. Thus, many routine microbiological approaches have not been applied, and the quality of information concerning the physiology and biochemistry of methanogens is much poorer than in other physiological groups where genetic techniques are commonly utilized. Previous work on the methanococci has addressed this problem with some success. These methanogens were among the first to be reliably and quantitatively enumerated by plating (Jones et al., 1983b). Strains carrying plasmids and phages were isolated and identified (Wood et al., 1985, 1989; Whitman et al., 1986). Methods were developed for enrichment of auxotrophic mutants similar to the penicillin selection in bacteria (Ladapo and Whitman, 1990). A selectable resistance marker called the pac cassette was created from the Streptomyces puromycin transacetylase gene and methanococcal transcriptional control elements (Gernhardt et al., 1990). A neomycin resistance cassette was also developed to provide a second selectable marker (Argyle et al., 1996). Several efficient transformation systems were developed (Bertani and Baresi, 1987; Micheletti et al., 1991; Patel et al., 1994; Tumbula et al., 1994). An expression vector was developed for Methanococcus maripaludis (Tumbula et al., 1997; Gardner and Whitman, 1999). Several reporter genes were developed (Beneke et al., 1995). The genomic sequence of M. maripaludis S2, a robust N 2 -fixing strain, was completed (Hendrickson et al., 2004). A system was developed for constructing markerless deletions (Moore and Leigh, 2005). Thus, the methanococci currently have one of the best developed genetic systems of any methanogen or archaeon. The rapid and reliable growth characteristics of the mesophilic methanococci are in part responsible for their success as experimental organisms. Methanogens typically grow slowly compared to many model microorganisms, such as E. coli . However, the mesophilic methanococci grow unusually fast for methanogens, with generation times of about two hours at 35-40 C (Jones et al., 1983a) . For liquid cultures, dense cultures can be obtained over night. Useful colonies typically grow on plates within three to four days. As for all methanogens anaerobic procedures are necessary, but efficient approaches have been well established for decades (Balch et al., 1979) . Importantly, M. maripaludis is also amenable to large-scale cultivation. Thus, it is possible to obtain 100 g to kg amounts of cells for biochemical experiments (Ibba et al., 1997) . Growth in chemostats is also possible (Haydock et al., 2004). Thus, it is possible to augment genetic studies with a wide variety of physiological and biochemical experiments. Methanococcus maripaludis strain C7: Although this strain is nutritionally similar to the type strain JJ, it only possesses 62 % DNA:DNA hybridization (S1 nuclease method) and represents a separate genospecies (Keswani et al., 1996). Similarly, it possesses 67 and 74 % DNA:DNA hybridization to strains C5 and C6, respectively, two other related genospecies. Unlike many strains of M. maripaludis , it grows well at low (0.06 M) concentrations of NaCl (Whitman et al., 1986). Otherwise, its nutritional requirements and morphology are similar to other members of the species. The mol % G+C is 33.7.
Bibliography Argyle, J. L., D. L. Tumbula, and J. A. Leigh. 1996. Neomycin resistance as a selectable marker in Methanococcus maripaludis . Appl Environ Microbiol 62:4233-7. Balch, W.E. and Wolfe, R.S. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethane-sulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32: 781-791. Balch, W.E., Fox, C.E., Magrum, L.J., Woese, C.R. and Wolfe R.S. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43: 260-296. Beneke, S., H. Bestgen, A. Klein. 1995. Use of the Escherichia coli uidA gene as a reporter in Methanococcus voltae for the analysis of the regulatory function of the intergenic region between the operons encoding selenium-free hydrogenases. Mol. Gen. Genet. 248: 225-228. Bertani, G., and L. Baresi. 1987. Genetic transformation in the methanogen Methanococcus voltae PS. J. Bacteriol. 169: 2730-2738. Bock, A. and Kandler, O. 1985. Antibiotic sensitivity of archaebacteria. In Woese, C.R. and Wolfe, R.S. (Editors), The Bacteria, Vol. VIII. Academic Press, New York, pp. 525-544. Boopathy, R. and Daniels, L. 1991. Pattern of organotin inhibition of methanogenic bacteria. Appl. Environ. Microbiol. 57: 1189-1193. Boopathy, R. and Daniels, L. 1992. Isolation and characterization of a marine methanogenic bacterium from the biofilm of a shiphull in Los Angeles Harbor. Curr. Microbiol. 25: 157-164. Corder, R.E., Hook, L.A., Larkin, J.M. and Frea, J.I. 1983. Isolation and characterization of two new methane-producing cocci: Methanogenium olentangyi , sp. nov., and Methanococcus deltae , sp. nov. Arch. Microbiol. 134: 28-32. Elhardt, D., and Bock, A. 1982. An in vitro polypeptide synthesizing system from methanogenic bacteria: sensitivity to antibiotics. Mol. Gen. Genet. 188: 128-134. Gardner, W.L., and W.B. Whitman. 1999. Expression vectors for Methanococcus maripaludis : overexpression of acetohydroxyacid synthase and $ -galactosidase. Genetics 152: 1439-1447. Gernhardt, P., O. Possot, M. Foglino, L. Sibold, and A. Klein. 1990. Construction of an integration vector for use in the archaebacterium Methanococcus voltae and expression of a eubacterial resistance gene. Mol. Gen. Genet. 221: 273-279. Haydock, A.K., I. Porat, W.B. Whitman, and J.A. Leigh (2004) Continuous culture of Methanococcus maripaludis under defined nutrient conditions. FEMS Microbiol. Lett. 238: 85-91. Hendrickson, E.L., R. Kaul, Y. Zhou, D. Bovee, P. Chapman, J. Chung, E. Conway de Macario, J.A. Dodsworth, W. Gillett, D.E. Graham, M. Hackett, A.K. Haydock, A. Kang, M.L. Land, R. Levy, T.J. Lie, T.A. Major, B.C. Moore, I. Porat, A. Palmeiri, G. Rouse, C. Saenphimmachak, D. Söll, S. Van Dien, T. Wang, W.B. Whitman, Q. Xia, Y. Zhang, F.W. Larimer, M.V. Olson and J.A. Leigh (2004) Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis. J. Bacteriol. 186: 6956-6969. Ibba, M., S. Morgan, A. W. Curnow, D. R. Pridmore, U. C. Vothknecht, W. Gardner, W. Lin, C. R. Woese, and D. Soll. 1997. A euryarchaeal lysyl-tRNA synthetase: resemblance to class I synthetases. Science 278:1119-22. Jones, J.B. and Stadtman, T.C. 1977. Methanococcus vannielii : culture and effects of selenium and tungsten on growth. J. Bacteriol. 130: 1404-1406. Jones, J.B., Bowers, B. and Stadtman, T.C. 1977. Methanococcus vannielii : ultrastructure and sensitivity to detergents and antibiotics. J. Bacteriol. 130: 1357-1363. Jones, W.J., Paynter, M.J.B. and Gupta, R. 1983a. Characterization of Methanococcus maripaludis sp. nov., a new methanogen isolated from salt marsh sediment. Arch. Microbiol. 135: 91-97. Jones, W.J., Whitman, W.B., Fields, R.D. and Wolfe, R.S. 1983b. Growth and plating efficiency of methanococci on agar media. Appl. Environ. Microbiol. 46: 220-226. Keswani, J., Orkand, S., Premachandran, U., Mandelco, L., Franklin, M.J. and Whitman, W.B. 1996. Phylogeny and taxonomy of mesophilic Methanococcus spp. and comparison of rRNA, DNA hybridization, and phenotypic methods. Int. J. Syst. Bacteriol. 46: 727-735. Kneifel, H., Stetter, K.O., Andreesen, J.R., Wiegel, J., Konig, H. and Schoberth, S.M. 1986. Distribution of polyamines in representative species of archaebacteria. System. Appl. Microbiol. 7: 241-245. Koga, Y., Morii, H., Akagawa-Matsushita, M. and Ohga, M. 1998. Correlation of polar lipid composition with 16S rRNA phylogeny in methanogens. Further analysis of lipid component parts. Biosci. Biotechnol. Biochem. 62: 230-236. Konig, H., Nusser, E. and Stetter, K.O. 1985. Glycogen in Methanolobus and Methanococcus . FEMS Microbiol. Lett. 28: 265-269. Ladapo, J. and W. B. Whitman (1990) Method for isolation of auxotrophs in the methanogenic archaebacteria: role of the acetyl-CoA pathway of autotrophic CO 2 fixation in Methanococcus maripaludis . Proc. Natl. Acad. Sci. USA: 87 :5598-5602. Micheletti, P.A., K.A. Sment, and J. Konisky. 1991. Isolation of a coenzyme M-auxotrophic mutant and transformation by electroporation in Methanococcus voltae . J. Bacteriol. 173: 3414-3418. Moore, B.C., and J.A. Leigh. 2005. Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J Bacteriol 187: 972-979. Patel, G.B., J.H.E. Nash, B.J. Agnew, and G.D. Sprott. 1994. Natural and electroporation-mediated tranformation of Methanococcus voltae . Appl. Environ. Microbiol. 60: 903-907. Rajagopal, B.S. and Daniels, L. 1986. Investigation of mercaptans, organic sulfides, and inorganic sulfur compounds as sulfur sources for the growth of methanogenic bacteria. Curr. Microbiol. 14: 137-144. Robertson, D.E., Roberts, M.F., Belay, N., Stetter, K.O. and Boone, D.R. 1990. Occurrence of $-glutamate, a novel osmolyte, in marine methanogenic bacteria. Appl. Environ. Microbiol. 56: 1504-1508. Tumbula, D.L., R.A. Makula, and W.B. Whitman. 1994. Transformation of Methanococcus maripaludis and identification of a Pst I-like restriction system. FEMS Microbiol. Lett. 121: 309-314. Tumbula, D.L., T.L. Bowen, and W.B. Whitman. 1997. Characterization of pURB500 from the archaeon Methanococcus maripaludis and construction of a shuttle vector. J. Bacteriol. 179: 2976-2986. Whitman, W.B. 1989. Order II. Methanococcales. In Staley, J.T., Bryant, M.P., Pfennig, N., and Holt, J.G. (Editors), Bergey's Manual of Systematic Bacteriology, 1 st Ed., Vol. 3, The Williams & Wilkins Co., Baltimore, pp. 2185-2190. Whitman, W.B., Ankwanda, E. and Wolfe, R.S. 1982. Nutrition and carbon metabolism of Methanococcus voltae . J. Bacteriol. 149: 852-863. Whitman, W.B., Shieh, J., Sohn, S., Caras, D.S. and Premachandran, U. 1986. Isolation and characterization of 22 mesophilic methanococci. Syst. Appl. Microbiol. 7: 235-240. Whitman, W.B., Sohn, S., Kuk, S., and Xing, R. 1987. Role of amino acids and vitamins in nutrition of mesophilic Methanococcus spp. Appl. Environ. Microbiol. 53: 2373-2378. Wood, A. G., W. B. Whitman and J. Konisky (1985) A newly-isolated marine methanogen harbors a small cryptic plasmid. Arch. Microbiol. 142 :259-261. Wood, A.G., W.B. Whitman, and J. Konisky. 1989. Isolation and characterization of an archaebacterial virus-like particle from Methanococcus voltae A3. J. Bacteriol. 171: 93-98. Zellner, G., and Winter. J. 1987. Secondary alcohols as hydrogen donors for CO 2 -reduction by methanogens. FEMS Microbiol. Lett. 44: 323-328.
|
||||
|
||||
Methanococcus maripaludis C7